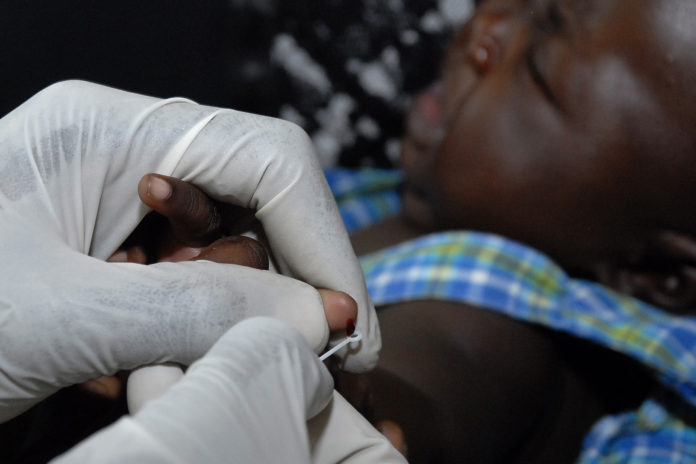
En réponse à la forte demande d’obtention du premier vaccin contre le paludisme, recommandé par l’Organisation Mondiale de la Santé, 12 pays d’Afrique se verront attribuer un total de 18 millions de doses pour la période 2023-2025. Ce déploiement est une étape cruciale dans la lutte contre l’une des maladies les plus meurtrières en Afrique, tuant près d’un demi-million d’enfants de moins de 5 ans et représentant environ 95 % des cas de cette maladie infectieuse dans le monde.
Depuis que l’Organisation Mondiale de la Santé a recommandé l’utilisation du premier vaccin antipaludique, RTS,S/AS01, pour la prévention du paludisme à Plasmodium falciparum chez les enfants vivant dans des régions où la transmission est modérée à élevée, on assiste à une très forte demande de doses.
Demande élevée
En juillet 2022, Gavi, l’Alliance du Vaccin, a proposé un financement pour aider les pays éligibles à déployer ce vaccin (et d’autres vaccins antipaludiques dès qu’ils seront disponibles). Depuis lors, plus de 28 pays ont exprimé leur intérêt pour obtenir le vaccin. Quatorze demandes, soumises lors des deux premières opportunités de candidature (septembre 2022 et janvier 2023), ont été recommandées pour approbation par le Comité d’examen indépendant (CEI) de Gavi. Actuellement, 18 millions de vaccins sont disponibles pour la période 2023-2025. Ce nombre est insuffisant pour permettre une distribution dans tous les pays recommandés par le CEI.
Phases-pilote
Depuis 2019, le Ghana, le Kenya et le Malawi administrent le vaccin antipaludique dans le cadre du Programme de mise en œuvre du vaccin antipaludique (MVIP), coordonné par l’OMS et financé par Gavi, le Fonds mondial de lutte contre le sida, la tuberculose et le paludisme, et Unitaid. Ainsi, le vaccin RTS,S/AS01 a été administré à plus de 1,7 million d’enfants dans les trois pays cités. Il s’est avéré sûr et efficace, entraînant à la fois une réduction substantielle du paludisme grave et une baisse du nombre de décès d’enfants.
Cadre d’allocation de vaccins
En prévision de la différence entre la demande et l’offre, l’OMS avait coordonné, début 2022, l’élaboration d’un cadre d’allocation pour attribuer de manière transparente une quantité limitée de vaccins antipaludiques. Le groupe de mise en œuvre du cadre d’allocation, composé de personnel technique de l’OMS, de l’UNICEF, du secrétariat de Gavi et des centres africains de contrôle et de prévention des maladies (Africa CDC), a été chargé de recommander les quantités de vaccins antipaludiques à allouer aux pays. La démarche a été effectuée en suivant les principes, les considérations et les indicateurs définis par le cadre d’allocation à savoir: un besoin urgent, un impact maximum sur la santé, un partage équitable des avantages et l’assurance de poursuite du programme de vaccination.
Prochaines vaccinations
Outre le Ghana, le Kenya et le Malawi, l’allocation initiale de 18 millions de doses permettra à neuf autres pays (Bénin, Burkina Faso, Burundi, Cameroun, République démocratique du Congo, Liberia, Niger, Sierra Leone, Ouganda) d’introduire pour la première fois le vaccin dans leurs programmes de vaccination systématique. Ce cycle d’attribution utilise les doses de vaccin dont dispose Gavi, par l’intermédiaire de l’UNICEF. Les premières doses de vaccin devraient arriver dans les pays au cours du dernier trimestre de 2023, et les pays devraient commencer à les administrer au début de 2024.
D’autres vaccins en dévelopement
La demande mondiale annuelle de vaccins antipaludiques est estimée à 40-60 millions de doses pour la seule année 2026, et passera à 80-100 millions de doses par an d’ici 2030. Outre le vaccin RTS,S/AS01, développé et produit par GSK et fourni à l’avenir par Bharat Biotech, un deuxième vaccin, R21/Matrix-M, développé par l’Université d’Oxford et fabriqué par le Serum Institute of India (SII), devrait également être bientôt préqualifié par l’OMS. Gavi a récemment présenté sa feuille de route pour soutenir l’augmentation de l’offre afin de répondre à la demande.